Valley
Fever--
Sneaky fungus that can make you very sick.
Synopses Coccidioidomycosis: A Reemerging Infectious
Disease Theo N. Kirkland, M.D., and Joshua Fierer, M.D. Departments of
Pathology and Medicine, University of California, San Diego School of Medicine
Department of Veterans Affairs Medical Center, San Diego, California, USA
Coccidioides immitis, the primary pathogenic fungus that causes coccidioidomycosis,
is most commonly found in the deserts of the southwestern United States and Central
and South America. During the early 1990s, the incidence of coccidioidomycosis
in California increased dramatically. Even though most infections are subclinical
or self-limited, the outbreak is estimated to have cost more than $66 million
in direct medical expenses and time lost from work in Kern County, California,
alone. In addition to the financial loss, this pathogen causes serious and life-threatening
disseminated infections, especially among the immunosuppressed, including AIDS
patients. This article discusses factors that may be responsible for the increased
incidence of coccidioidomycosis (e.g., climatic and demographic changes and the
clinical problems of coccidioidomycosis in the immunocompromised) and new approaches
to therapy and prevention. Emerging infectious diseases have been defined
as “infections that have newly existed in a population or have existed but
are rapidly increasing in incidence or geographic range” (1). In what sense
is coccidioidomycosis an emerging infectious disease? Coccidioidomycosis is not
a new disease; it was first recognized and reported slightly more than 100 years
ago by a medical student in Argentina (2). In fact, coccidioidomycosis has affected
inhabitants of the desert Southwest for thousands of years (3). However, in the
past several years, the number of cases of coccidioidomycosis has increased dramatically,
and the clinical symptoms of this illness have changed in patients with acquired
immunodefficiency syndrome (AIDS). In this article, we explore some of the reasons
for the increased incidence of coccidioidomycosis, review the new clinical data,
and discuss current approaches to therapy and prevention. 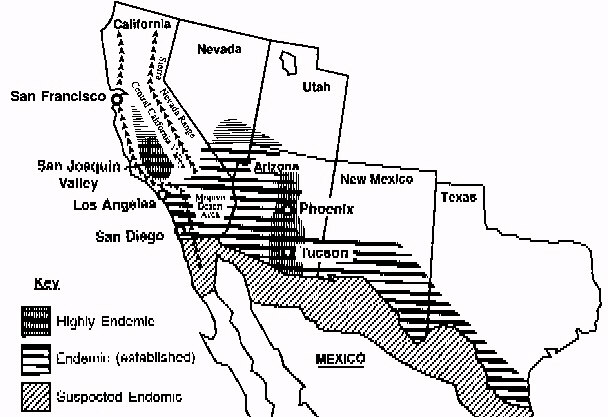
Etiology Coccidioidomycosis is caused by Coccidioides immitis,
a dimorphic fungus that grows as a mold in the soil. The mold forms arthroconidia
within the hypha, a type of conidia formation known as enteroarthric development
(Figure 1) [Figures not available in ASCII] (4). C. immitis is the only species
within the primary pathogenic fungi that has this type of conidia development.
Alternate conidia undergo autolysis, leaving empty spaces between viable arthroconidia.
The arthroconidia are released into the atmosphere when the wind ruptures the
hypha. C. immitis infects humans and animals almost exclusively by the respiratory
route (5). Once inhaled, the arthroconidia cluster in the lungs and undergo a
dramatic morphologic change. The round cells, which develop into spherules, undergo
repeated internal divisions until they are filled with hundreds to thousands of
offspring, termed endospores. This process occurs over 48 to 72 hours (6). When
the spherule ruptures, each released endospore has the capacity to develop into
a mature spherule. Epidemiology C. immitis is primarily found
in desert soil. It is present in highest numbers in the San Joaquin Valley in
California, southern Arizona, southern New Mexico, west Texas, and the desert
areas of northern Mexico (Figure 2) [Figures not available in ASCII]. The organism
is also found in scattered foci in coastal southern California, southern Nevada,
and Utah (7) and is endemic in a few areas in Central and South America, especially
in Venezuela (7). C. immitis is distributed unevenly in the soil and seems to
be concentrated around animal burrows and ancient Indian burial sites (8,9); it
is usually found 4 to 12 inches below the surface of the soil (7). Since C.
immitis infects humans by the respiratory route, exposure to dust is one critical
factor determining the risk for infection (10). Coccidioidomycosis is not spread
from person to person, except in extraordinary circumstances. Coccidioidomycosis
probably had its most profound effect on the population of the United States during
World War II when several training airfields were built in the San Joaquin Valley.
The rate of new infections in military personnel was 8% to 25% per year (10).
Coccidioidomycosis was the most common cause of hospitalization at many airbases
in the Southwest. Though the death rate was very low, many soldiers were sick
for weeks to months, and their training was completely disrupted. At least in
part because of efforts to minimize dust, the infection rate declined as the war
went on (10). The incidence of coccidioidomycosis varies with the season;
it is highest in late summer and early fall when the soil is dry and the crops
are harvested (10). If it rains at this time of the year (which is unusual in
southern California), disease incidence declines as the amount of dust decreases.
Dust storms are frequently followed by outbreaks of coccidioidomycosis. One particularly
severe dust storm in 1977 carried dust from the San Joaquin Valley up to the San
Francisco Bay area and resulted in hundreds of cases of nonendemic coccidioidomycosis
in areas north of the San Joaquin Valley (11). More recently, an earthquake centered
in Northridge, California, was associated with 170 cases of acute coccidioidomycosis
in Ventura County, which normally has a low incidence of this disease. The airborne
dust associated with landslides triggered by the earthquake was implicated in
the increase in the number of cases (12). Occupational or recreational exposure
to dust is also an important consideration. Agricultural workers, construction
workers, or others (such as archeologists) who dig in the soil in the disease-endemic
area are at increased risk for the disease (13,14). During World War II, C. E.
Smith, one of the most perceptive and influential epidemiologists to study coccidioidomycosis,
recommended dust control as a primary measure to reduce risk for exposure (10).
However, because the desert is inherently dusty, many cases of coccidioidomycosis
are acquired just by driving through the disease-endemic area. Clinical
Illness C. immitis is transmitted by the respiratory route. Smith et
al., in a prospective study of cases of coccidioidomycosis acquired during World
War II by soldiers at three San Joaquin Valley airbases, skin-tested the airmen
periodically and questioned them about illnesses in the interval. They found that
most infections (60%) were asymptomatic and resolved spontaneously; 15% were not
severe enough to require medical care, and 25% were clinically important and required
a substantial amount of time off work (15). In symptomatic patients, the pulmonary
illness ranges from a self-limited flulike illness to pneumonia (16). Approximately
5% of primary infections result in erythema nodosum or erythema marginatum with
associated noninfectious arthritis; most of those patients have a self-limited
infection (17). Particularly in persons with diabetes, multiple thin-walled chronic
cavities tend to develop as a residual effect of pulmonary coccidioidomycosis
(18). Unlike in tuberculosis, in coccidioidomycosis, dissemination almost always
becomes evident within a few weeks of the primary pneumonia, although in cases
of limited dissemination it may not become clinically evident until months later
(15,19). Cocidioidomycosis can disseminate and cause miliary disease, bone and
joint infection, skin disease, soft tissue abscesses, and meningitis (15,16).
These extrapulmonary complications are uncommon (-5% of infections). The risk
for disseminated coccidioidomycosis is much higher among some ethnic groups, particularly
African-Americans and Filipinos. In these ethnic groups, the risk for disseminated
coccidioidomycosis is tenfold that of the general population (5,20). Presumably,
a gene (or genes) that increases susceptibility to infection is more prevalent
in these ethnic groups than in the general population. Such a resistance gene
has been identified in mice (21-23), but not yet in humans. The mechanism by which
the resistance genes affect the course of the disease in mice is not clear. Pregnant
women and the immunosuppressed are also at high risk for developing disseminated
disease (Figure 3) [Figures not available in ASCII] (24). One study demonstrated
that the growth rate of spherules was influenced by human sex hormones, which
may partially account for the increased risk of disseminated disease in pregnancy
(25). Pregnancy also redirects the immune response toward humeral (TH2) immunity
and away from delayed hypersensitivity (TH1) (26), which may influence resolution
of coccidioidomycosis. Generalized suppression of cell mediated immunity also
increases the risk of disseminated disease (27). Coccidioidomycosis is particularly
severe in patients with organ transplants or AIDS. Though disseminated coccidioidomycosis
is uncommon, and symptomatic coccidioidal pneumonia usually resolves without therapy,
many of these patients are very ill for weeks to months. Galgiani reported that
a group of college students in Tucson who had coccidioidomycosis required an average
of six clinic visits before the disease resolved (16). Therefore, this can be
an expensive illness in terms of medical costs and time lost from work or school,
even when the infection resolves spontaneously. Coccidioidomycosis Epidemic in
California Kern County, in the San Joaquin Valley, California, is one of the
most highly coccidioidomycosis-endemic regions. The number of new cases of coccidioidomycosis
in the area has varied widely from year to year; a low incidence of coccidioidomycosis
from 1987 to 1990 (-500 reported cases a year in Kern County), was followed by
a high incidence from 1991 to 1994 (28-30). The number of reported cases, which
were identified by serologic testing at the Kern County Health Department (the
reference serology laboratory for the county), probably represent approximately
10% of the total number of infected persons in that county (Figure 4) [Figures
not available in ASCII] (28). The medical costs for infected persons in Kern County
are estimated at $66 million (29). In 1992, 4,500 new cases were reported to the
California State Department of Health Services (30), most from Kern County; the
number of new cases also increased in almost all counties in central and southern
California (30). The increase in reported cases in California in 199192 was dramatic
but certainly an underestimate of the magnitude of the problem (31). The epidemic
seems to be waning, for reasons that are not clear, but the marked increase in
incidence from the 1980s to 1991 through 1993 is indisputable. What factors may
account for this increase? One major consideration is the weather. C. E. Smith
observed years ago that the number of cases of coccidioidomycosis was higher in
the summer after a rainy winter than after a dry winter (10). In March 1991, a
5-year drought in California ended with a heavy rainfall. Rainfall was also heavy
in the winters of 1992 and 1993. Though the relationship between the weather and
the density of C. immitis in the soil may never be understood in detail, the following
scenario seems plausible. During drought years, the number of organisms competing
with C. immitis decreases. C. immitis does not thrive, but it remains viable though
dormant. After heavy rain, the arthrocondia germinate and multiply to a higher
density than usual because of the lack of competing organisms. Once the soil dries
in the late summer and fall, the arthroconidia become airborne and potentially
infectious (29). Another reason for the sudden increase in disease incidence
might have been the number of susceptible persons in the disease-endemic area.
The number may have been the result of both increased migration of susceptible
persons and decreased immunity in the indigenous population. Immunity comes from
prior infection and is manifest as a positive coccidioidin skin test. In almost
all cases, coccidioidomycosis confers lifelong immunity. As a result of years
of low incidence, the number of nonimmune persons may have increased, as evidenced
by the decrease in prevalence of positive coccidioidin skin tests among local
high school students. In 1939, 50% to 60% of high school students in the San Joaquin
Valley had positive skin tests (17), but in the 1980s only 3% to 5% of high school
students had positive skin tests (T. Larwood, pers. comm.). Given the historical
data, this estimate seems low, but another study also found a low prevalence.
In 1985, workers in Tucson estimated that 30% of a random sample of persons in
a Hispanic neighborhood had positive skin tests (32). In addition to the drought,
irrigation of fields, the increasing amount of land under cultivation, and a decrease
in indoor dust due to the widespread use of air conditioning may also have played
a role in the relatively low incidence of infections in the 1980s. Coccidioidomycosis
in the Immunosuppressed C. immitis is a primary pathogen that can cause
disease in immunologically healthy persons. In the population as a whole, fewer
than 5% of infected persons have persistent pulmonary infection or extrapulmonary
dissemination of the disease (16). The incidence of clinically significant disease
in immunosuppressed patients is much higher. In one study symptomatic coccidioidomycosis
developed in 18 (7%) of 260 renal transplant patients in Arizona over a 10-year
period, primarily in the first year after transplantation (33). This rate was
substantially higher than the rate of infection in patients who were undergoing
hemodialysis. Approximately 12 (67%) of infections in the patients with renal
transplants were disseminated; the remainder were confined to the lung. Of patients
with disseminated disease, 10 (83%) died, despite intensive therapy with amphotericin
B. In another study from Tucson, all confirmed cases of coccidioidomycosis during
a 4-year period were reviewed. The dissemination rate was 8 (73%) of 11 of patients
who were receiving immunosuppressive therapies, compared with only 15 (14%) of
110 healthy controls (34). As more patients in the disease-endemic area receive
liver, lung, and heart transplants, this problem will increase. Pregnant women,
especially those in the third trimester, are at high risk for developing disseminated
coccidioidomycosis if they become infected (24). In the first and second trimesters,
the risk is much lower. The reason is not entirely clear, but two factors may
play a role: 1) the high sex hormone levels found in late pregnancy enhance the
growth of C. immitis in vitro (25), and 2) the shift in the T-cell immune response
late in pregnancy toward TH2 cytokines (26) interferes with resolving the infection.
In experimental animals, pregnancy increases the severity of leishmaniasis, another
infection hat is controlled by a TH1 T-cell response (35). Coccidioidomycosis
in AIDS patients is also very likely to be life-threatening. The first cases of
coccidioidomycosis described in AIDS patients were atypical, with a reticulonodular
chest x-ray pattern, positive blood cultures, and infection of multiple organs
(36). As we have gained more experience with coccidioidomycosis in HIV-infected
persons, we have learned that the clinical spectrum is broader than originally
reported. Fish and his colleagues collected data from 77 AIDS patients with coccidioidomycosis
who were treated by physicians in Arizona and California (37). They grouped the
patients according to their clinical symptoms (Figure 5) [Figures not available
in ASCII]. Although the largest group of patients had diffuse pulmonary infiltrates,
a significant fraction had focal pulmonary disease, meningitis, or other extrapulmonary
disease. Six patients had only a positive serologic test with no other evidence
of infection. Excluding the patients who had only a positive serologic test, 81%
of the patients in this series had a positive serologic test for coccidioidomycosis,
either for IgM or IgG antibodies. However, only 69% of patients with diffuse pulmonary
disease had a positive serologic test, and the death rate in this group was also
the highest (70%). In all clinical groups, death was correlated with the number
of circulating CD4 T-cells at the time of diagnosis. For clinicians, however,
the most important message from this study is that coccidioidomycosis is not a
uniformly fatal complication in patients with AIDS, and that many forms of this
disease, including meningitis, respond to therapy. Patients with -200 CD4 T-cells/µl
are more likely to have severe, disseminated infections. A more recent prospective
study of 170 HIV-infected persons in an area of Arizona where coccidioidomycosis
is endemic showed a cumulative incidence of coccidioidomycosis of 25% over 41
months (38). The most important risk factors were the level of CD4 T-cells and
the diagnosis of AIDS (as opposed to HIV infection). HIV-infected patients with
AIDS or -250 CD4 T-cells/µl were 8 to 35 times more likely to get coccidioidomycosis.
History of coccidioidomycosis, a history of a positive skin test for coccidioidomycosis,
or a prolonged stay in the disease-endemic area were not associated with an increased
risk for infection. These data suggest that most cases were primary infections
in severely immunosuppressed patients. Since patients with AIDS were not more
likely to be exposed to the spores of C. immitis and all patients were seen prospectively
at 4-month intervals and tested for antibody to C. immitis, severe immunosuppression
appeared to increase their risk for infection, as well as disease. As in the retrospective
study reviewed above, the clinical symptoms varied widely, ranging from mild to
extremely severe. Only one patient had antibody titers to C. immitis by complement
fixation test without any other evidence of disease. Treatment
Various drugs are now available for treating coccidioidomycosis. In addition to
amphotericin B, which must be given intravenously and is considerably toxic, triazole
compounds have been found to be active agents for treating most manifestations
of coccidioidomycosis. Fluconazole, in an uncontrolled study, was reported to
be effective primary therapy for coccidioidal meningitis; since untreated coccidioidal
meningitis is uniformly fatal, robust conclusions could be drawn from this trial
(39). Forty-seven consecutive patients were treated with 400 mg/day of fluconazole;
during the first 6 months of therapy, 33 (70%) of the patients responded to therapy.
(A response was defined as a 40% reduction in a score, on the basis of clinical
measurements and cerebrospinal fluid findings.) Two patients who did not respond
to therapy died of coccidioidomycosis; both were HIV-positive. Because of previous
experience with high relapse rates when azole therapy is stopped, the authors
recommended lifelong treatment with fluconazole. In a small study, four of five
patients treated for meningitis with itraconazole as sole therapy responded favorably
(40). A recent article emphasized the high relapse rate after azole therapy is
stopped (41). The alternative treatment to the azoles is amphotericin B. If amphotericin
B is used to treat meningitis, however, it must be given intrathecally as well
as intravenously, and this greatly increases the risk for a toxic reaction to
that drug. Clearly, fluconazole and itraconazole can be used to treat patients
with nonmeningeal coccidioidomycosis (42-44). Whether one of these drugs is superior
to the other, or how either one compares to amphotericin B is not known. It seems
prudent to treat extremely ill patients with amphotericin B, at least until their
clinical situation stabilizes, although no published studies support that point
of view. However, few (if any) patients with the acute miliary form of coccidioidomycosis
have been included in any of the reported studies of any of the azole drugs. New
agents that are more active against coccidioidomycosis are still sorely needed.
Prevention Simple environmental measures, such as planting grass
or paving roads in highly populated areas, decrease the amount of airborne dust
and lower the risk for coccidioidomycosis (10). These measures do not necessarily
eradicate C. immitis from the soil but lower the risk for airborne dispersion
of the organism. At present, no practical method exists for eliminating C. immitis
from the soil. Vaccine Development An alternative approach is
to vaccinate persons at risk. A vaccine is feasible because natural infection
almost always confers lifelong immunity from reinfection. Furthermore, good animal
models exist to test vaccine candidates (21). Finally, genetically susceptible
mice can be successfully immunized, which suggests that the genetically susceptible
human population would also benefit from vaccination (21). One vaccine that
has been tested is a killed spherule vaccine developed by Pappagianis and Levine.
It protected mice and other animals from experimental infection with C. immitis
(45). Between 1980 and 1985, a double-blinded human study compared results of
a formalin-killed spherule vaccine with results obtained from a placebo. In this
study, which involved almost 3,000 people, only a minority of the vaccinated persons
had positive skin test results to C. immitis. Although the incidence of coccidioidomycosis
was low while this study was conducted, no difference was found in the number
of cases of coccidioidomycosis or the severity of the disease in the vaccinated
group compared with that for the placebo-receiving control group (46). One explanation
for the ineffectiveness of this vaccine may be that relatively small numbers of
killed organisms could be injected into human without unacceptable local side
effects of pain and swelling. Nevertheless, the vaccine trial made it clear that
immunization with tolerable numbers of whole killed-spherules does not provide
immunoprotection against coccidioidomycosis in humans. Since the cell wall
of C. immitis is made up primarily of nonprotein macromolecules, it contains a
large amount of material presumably nonantigenic for T lymphocytes. Therefore,
the whole organism is not the ideal vaccine candidate. Ideally, one would like
to vaccinate patients selectively only with antigens that stimulate a protective
T-cell-mediated immune response. These antigens have been difficult to identify,
and a consensus on what they are does not exist. Various approaches have been
used to obtain antigenic proteins. In one, a lysate of arthroconidia (coccidioidin)
or spherules (spherulin) was made (47). Alkali treatment has also been used to
extract antigens from arthroconidia and spherules (48). Another approach has been
to use C. immitis antigens obtained without extraction or autolysis. The advantage
of this method is that one should obtain reproducible preparations of intact proteins.
Cole and co-workers (49) found that when the outer conidial wall was removed from
arthroconidia, the organism released various proteins (called the soluble conidial
wall fraction). This mixture of proteins was extraordinarily effective in stimulating
the proliferation of C. immitis-immune T cells in mice. Another antigenic mixture
is a membranous material consisting primarily of proteins and lipids that the
spherule phase of the organism spontaneously releases (the spherule outer wall).
This spherule wall fraction has been shown to be an active antigen in T-cell-mediated
immune responses in mice (50). All of these mixtures are heterogeneous and
difficult to fractionate biochemically. This is probably due, at least in part,
to differences in glycosylation, which makes physically separating the proteins
difficult. To resolve this problem, Galgiani and his colleagues deglycosylated
the proteins from a toluene spherule lysate by using hydrogen fluoride (51,52).
Although this treatment does remove all sugars, it is extraordinarily harsh and
yields less than 10% of the initial protein, with most of the protein forming
an insoluble precipitate. Nevertheless, the resulting product reacts with reference
antiserum to C. immitis in immunoelectrophoresis. This antigen also stimulated
a proliferative T-cell response in patient lymphocytes but not in those of the
control group (noninfected donors). Another way to attack the problem of generating
pure C. immitis antigens is to use molecular biologic techniques. The advantage
to this approach is that once antigens are molecularly cloned, and the protein
is expressed, an essentially unlimited source of completely defined antigen is
available. Therefore, one would not have to repeatedly grow C. immitis, extract
the antigen, and purify it from a complex mixture. In addition, with the molecular
approach, antigens could be delivered as part of a living vaccine system, should
that be required to effectively immunize people against coccidioidomycosis. We
believe that systematically identifying and evaluating C. immitis T-cell reactive
antigens in experimental animals is a rational approach to the ultimate development
of a vaccine. Our laboratories, in collaboration with Garry Cole, have used a
murine T-cell line that is specific for soluble conidial wall fraction antigens
to identify one cloned fragment of a C. immitis protein (53). Recently, genomic
DNA clones coding for this protein have been identified and sequenced. Significant
homology exists between this C. immitis antigen and the human enzyme 4 hydroxyphenylpyruvate
dioxygenase (54). This protein has been expressed in bacteria and was found to
elicit T-lymphocyte proliferative responses in mice immune to C. immitis. We are
testing its efficacy as an experimental vaccine. With the exception of alkali
extracted spherules (55) and whole killed spherules (45), none of the T-cell reactive
antigens have been shown to be immunoprotective in experimental models. However,
it is reasonable to expect that some antigen, or mixture of antigens, will be
found that can confer protective immunity in experimental animals. Molecular strategies
are available to accomplish this task and are an important area of future research.
Once a vaccine has been successfully tested in animals, another human vaccine
trial would be feasible. Dr. Kirkland is associate professor of pathology
and medicine; Dr. Fierer is professor of medicine and pathology and head of the
Division of Infectious Diseases, University of California, San Diego School of
Medicine. Drs. Kirkland and Fierer have worked together for the past 15 years.
Currently, they are focusing on the genetic determinants for resistance to infection
and on identifying candidates for a coccidioidomycosis vaccine. Acknowledgments
We are grateful to Dr. Ron Talbot, Director of the Kern County Public
Health Laboratory, and Dr. Tom Larwood for sharing unpublished data with us. The
experimental work done in our laboratories has been supported by National Institutes
for Health grants AI19149 and AI37232 and by the Research Service of the Department
of Veterans Affairs. Address for correspondence: Theo N. Kirkland, M.D., Department
of Pathology and Medicine, University of California, San Diego School of Medicine,
Department of Veterans Affairs Medical Center (111-F) 3350 La Jolla Village Dr.,
San Diego, CA 92161, USA; fax: 619-552-4398; e-mail: tkirkland@ucsd.edu.
References 1. Morse SS. Factors in the emergence of infectious diseases.
Emerging Infectious Diseases 1995;1:7-15.
2. Deresinski SC. History of coccidioidomycosis:
“dust to dust.” In: Stevens DA, editor. Coccidioidomycosis. New York:
Plenum, 1980:1-20.
3. Harrison WR, Merbs CF, Leathers CR. Evidence of coccidioidomycosis
in the skeleton of an ancient Arizona Indian. J Infect Dis 1991;164:436-7.
4. Cole GT, Sun SH. Arthroconidium-spherule-endospore transformation in Coccidioides
immitis. In: Szaniszlo PJ, Harris L, editors. Fungal dimorphism: with emphasis
on fungi pathogenic for humans. New York: Plenum, 1985:281-333.
5. Pappagianis
D. Epidemiology of coccidioidomycosis. Curr Top Med Mycol 1988;2:199-238.
6. Converse JL. Effect of surface active agents on endosporulation of Coccidioides
immitis in a chemically defined medium. J Bacteriol 1957;74:106-7.
7. Kamel
SM, Wheat LJ, Garten ML, Bartlett MS, Tansey MR, Tewari RP. Production and characterization
of murine monoclonal antibodies to Histoplasma capsulatum yeast cell antigens.
Infect Immun 1989;57:896-901.
8. Lacy GH Swatek FE. Soil ecology of Coccidioides
immitis at Amerindian middens in California. Appl Microbiol 1974;27:379-88.
9. Maddy KT. The geographic distribution of Coccidioides immitis and possible
ecologic implications. Ariz Med 1958;15:178-88.
10. Smith CE, Beard RR, Rosenberger
HG, Whiting EG. Effect of season and dust control on coccidioidomycosis. JAMA
1946;132:833-8.
11. Pappagianis D, Einstein H. Tempest from Tehachapi takes
toll or Coccidioides conveyed aloft and afar. West J Med 1978;129:527-30.
12. CDC. Coccidioidomycosis following the Northridge earthquake—California,
1994. MMWR 1994;43:194-5.
13. Johnson WM. Occupational factors in coccidioidomycosis.
J Occup Med 1981;23:367-74.
14. Werner SB, Pappagianis D. Coccidioidomycosis
in northern California—an outbreak among archeology students near Red Bluff.
Calif Med 1973;119:16-20.
15. Smith CE, Beard RR, Whiting EG, Rosenberg HG.
Varieties of coccidioidal infection in relation to the epidemiology and control
of diseases. Am J Public Health 1946;36:1394-1402.
16. Galgiani JN. Coccidioidomycosis.
West J Med 1993;159:153-71.
17. Smith CE. Epidemiology of acute coccidioidomycosis
with erythema nodosum (“San Joaquin” or “Valley Fever”). Am
J Public Health 1940;30:600-11.
18. Baker EJ, Hawkins JA, Waskow EA. Surgery
for coccidioidomycosis in 52 diabetic patients with special reference to related
immunologic factors. J Thorac Cardiovasc Surg 1978;75:680-7.
19. Smith CE,
Beard RR, Saito MT. Pathogenesis of coccidioidomycosis with special reference
to pulmonary cavitation. Ann Intern Med 1948;29:623-55.
20. Williams PL,
Sable DL, Mendez P, Smyth LT. Symptomatic coccidioidomycosis following a severe
natural dust storm—an outbreak at the Naval Air Station, Lemoore, Calif.
Chest 1979;76:566-70.
21. Kirkland TN, Fierer J. Inbred mouse strains differ
in resistance to lethal Coccidiodes immitis infection. Infect Immun 1983;40:912.
22. Kirkland TN, Fierer J. Genetic control of resistance to Coccidioides
immitis: a single gene that is expressed in spleen cells determines resistance.
J Immunol 1985;135:548-52.
23. Cox RA, Kennell W. Suppression of T-lymphocyte
response by Coccidioides immitis antigen. Infect Immun 1988;56:1424-9.
24.
Wack EE, Ampel NM, Galgiani JN, Bronnimann DA. Coccidioidomycosis during pregnancy—an
analysis of ten cases among 47,120 pregnancies. Chest 1988;94:376-9.
25.
Powell BL, Drutz DJ, Huppert M, Sun SH. Relationship of progesterone- and estradiol-binding
proteins in Coccidioides immitis to coccidioidal dissemination in pregnancy. Infect
Immun 1983;40:478-85.
26. Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional
cytokine interactions in the maternal-fetal relationship: is successful pregnancy
a TH2 phenomenon? Immunol Today 1993;14:353-6.
27. Beaman L, Pappagianis
D, Benjamini E. Significance of T-cells in resistance to experimental murine coccidioidomycosis.
Infect Immun 1977;17:580-5.
28. Einstein HE, Johnson RH. Coccidioidomycosis:
new aspects of epidemiology and therapy. Clin Infect Dis 1993;16:349-54.
29. Jinadu BA. Valley Fever Task Force report on the control of Coccidioides immitis.Bakersfield,
CA: Kern Country Health Department, 1995.
30. Pappagianis D. Marked increase
in cases of coccidioidomycosis in California: 1991, 1992, and 1993. Clin Infect
Dis 1994;19:S14-8.
31. CDC. Update: Coccidioidomycosis--California, 19911993.
MMWR 1994;43:421-3.
32. Dodge RR, Lebowitz MD, Barbee R, Burrows B. Estimates
of C. immitis infection by skin test reactivity in an endemic community. Am J
Public Health 1985;75:863-5.
33. Cohen IM, Galgiani JN, Potter D, Ogden DA.
Coccidioimomycosis in renal replacement therapy. Arch Intern Med 1982;142:489-94.
34. Rutala PJ, Smith JW. Coccidioidomycosis in potentially compromised hosts:
the effect of immunosuppressive therapy in dissemination. Am J Med Sci 1978;275:283-95.
35. Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic
M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection
and causes decreased antigenspecific IFN-gamma response and increased production
of T helper 2 cytokines. J Immunol 1996;156:644-52.
36. Bronnimann DA, Adam
RD, Galgiani JN, Habib MP, Peterson EA, Porter B, et al. Coccidioidomycosis in
the acquired immunodeficiency syndrome. Ann Intern Med 1987;106:372-9.
37.
Fish DG, Ampel NM, Galgiani JN, Dols CL, Kelly PC, Johnson CH, et al. Coccidioidomycosis
during human immunodeficiency virus infection—a review of 77 patients. Medicine
(Baltimore) 1990;69:384-91.
38. Ampel NM, Dols CL, Galgiani JN. Coccidioidomycosis
during human immunodeficiency virus infection: results of a prospective study
in a coccidioidal endemic area. Am J Med 1993;94:235-40.
39. Galgiani JN,
Catanzaro A, Cloud GA, Higgs J, Friedman BA, Larsen RA, et al. Fluconazole therapy
for coccidioidal meningitis: the NIAID-Mycoses Study Group. Ann Intern Med 1993;119:28-35.
40. Tucker RM, Denning DW, Dupont B, Stevens DA. Itraconazole therapy for
chronic coccidioidal meningitis. Ann Intern Med 1990;112:108-12.
41. Dewsnup
DH, Galgiani JN, Leviner BE, Sharkey-Mathin PK, Fierer J, Stevens DA. Is it ever
safe to stop azole therapy for Coccidioides immitis meningitis? Ann Intern Med
1996;124:305-10.
42. Catanzaro A, Fierer J, Friedman PJ. Fluconazole in the
treatment of persistent coccidioidomycosis. Chest 1990;97:666-9.
43. Graybill
JR, Stevens DA, Galgiani JN, Dismukes WE, Cloud GA, et al. Itraconazole treatment
of coccidioidomycosis. Am J Med 1990;89:282-90.
44. Catanzaro A, Galgiani
JN, Levine BE, Sharkey-Mathis PK, Fierer J, Stevens DA, et al. Fluconazole in
the treatment of chronic pulmonary and nonmeningeal disseminated coccidioidomycosis.
NIAID Mycoses Study Group. Am J Med 1995;98:249-56.
45. Levine HB, Cobb JM,
Smith CE. Immunogenicity of spherule-endospore vaccines of Coccidioides immitis
for mice. J Immunol 1961;87:218-27.
46. Pappagianis D, et al. Evaluation
of the protective efficacy of the killed Coccidioides immitis spherule vaccine
in humans. Am Rev Respir Dis 1993;148:656-60.
47. Huppert M, Spratt NS, Vukovich
KR, Sun SH, Rice EH. Antigenic analysis of coccidioidin and spherulin determined
by two-dimensional immunoelectrophoresis. Infect Immun 1978;20:541-51.
48.
Cox RA, Britt LA. Isolation and identification of an exoantigen specific for Coccidioides
immitis. Infect Immun 1986;52:138-43.
49. Cole GT, Kirkland TN, Sun SH. An
immunoreactive, water-soluble conidial wall fraction of Coccidioides immitis.
Infect Immun 1987;55:657-67.
50. Cole GT, Kirkland TN, Zhu M, Yuan L, Sun
SH, Hearn VN. Immunoreactivity of a surface wall fraction produced by spherules
of Coccidioides immitis. Infect Immun 1988;56:2695-701.
51. Dugger KO, Galgiani
JN, Ampel NM, Sun SH, Magee DM, Harrison J, et al. An immunoreactive apoglycoprotein
purified from Coccidioides immitis. Infect Immun 1991;59:2245-51.
52. Galgiani
JN, Sun SH, Dugger KO, Ampel NM, Grace GC, Harrison J, et al. An arthroconidial
spherule antigen of Coccidioides immitis: differential expression during in vitro
fungal development and evidence for humoral response in humans after infection
or vaccination. Infect Immun 1992;60:2627-35.
53. Kirkland TN, Zhu SW, Cruse
D, Hsu LL, Seshan KR, Cole GT. Coccidioides immitis fractions which are antigenic
for immune T lymphocytes. Infect Immun 1991;59:3952-61.
54. Wycoff E, Pishco
J, Kirkland TN, Cole GT. Cloning and expression of a T-cell reactive protein from
Coccidioides immitis: homology to 4-hydroxyphenylpyruvate dixygenase and the mammalian
Fantigen. Gene 1995;161:107-11.
55. Lecara G, Cox RA, Simpson RB. Coccidioides
immitis vaccine: potential of an alkali-soluble, water-soluble cell wall antigen.
Infect Immun 1983;39:473-5.

BACK
TO ENTRY PAGE OF THE JOURNAL ge |